Key Concepts
- A molecule consists of 2 or more atoms joined by covalent bonds.
- The shape of a molecule is a description of the way the atoms in the molecule occupy space.
- A diatomic molecule, a molecule composed of only 2 atoms, must always be linear in shape as the centers of the 2 atoms will always be in a straight line.
- 'Electron Cloud' Repulsion Theory (Valence Shell Electron Pair Repulsion, VSEPR) is used to predict shapes and bond angles of simple molecules
- an 'electron cloud' may be a single, double or triple bond, or a lone pair of electrons
- a lone pair of electrons is a non-bonding pair of electrons
- 'electron clouds' are negatively charged since the electrons are negatively charged, so electron clouds repel one another and try to get as far away from each other as possible
- lone pairs of electrons exert a greater repelling effect than bonding pairs do
- lone pair-bonding pair repulsion is greater than bonding pair-bonding pair repulsion
- lone pair-lone pair repulsion > lone pair-bonding pair repulsion > bonding pair-bonding pair repulsion
| Total Number of electron pairs | Arrangement of electron pairs | Number of bonding pairs of electrons | Number of lone pairs of electrons | Shape of Molecule | Name of Shape | Bond Angle | Examples |
|---|
| not applicable | linear | 1 | not applicable | 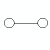 | linear | 180o | H2, HCl |
| 2 | linear | 2 | 0 | 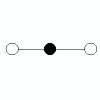 | linear | 180o | CO2, HCN |
| 3 | trigonal planar | 3 | 0 | 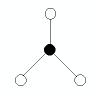 | triganol planar | 120o | BCl3, AlCl3 |
| 4 | tetrahedral | 4 | 0 | 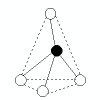 | tetrahedral | 109.5o | CH4, SiF4 |
| 3 | 1 | 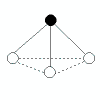 | trigonal pyramidal | <109.5o(bond angles in ammonia, NH3, are 107o) | NH3, PCl3 |
| 2 | 2 | 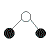 | bent | <109.5o(bond angles in water, H2O, are 105o) | H2O, SCl2 |
| 5 | trigonal bipyramidal | 5 | 0 | 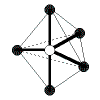 | trigonal bipyramidal | 120o in the trigonal planar part of the molecule, 90o for the others | PCl5 |
| 6 | octahedral | 6 | 0 | 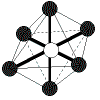 | octahedral | 90o | SF6
|
For More InFo Visit http://www.ausetute.com.au/shapemol.html |
You are a science genius! I already forgot all of these after I left college. Good for you!
ReplyDeleteThanku Superlux.Every One usually forget every thing after their collages.Because there is no use of that to remeber later.And lol I am a student of 16 years old.I use more time for reading books rather studying.Plz say which country are you from?
DeleteYou are always welcome to this blog my friend
science4keralasyllabus.blogspot.in